By Alex Berezow
Hope is beginning to fade that the world will have a safe and effective coronavirus vaccine before the predicted “second wave” arrives that will further suppress economic activity and recovery. Despite an unprecedented global effort, a deliverable vaccine might still be months away. Almost certainly it won’t be ready by October as many hoped. Dr. Anthony Fauci, head of the U.S. National Institute of Allergy and Infectious Diseases, said November or December would be more realistic.
It is understandable that governments and politicians are trying to be optimistic. The global economy was pummeled in the first wave, and it is hardly in shape to receive another devastating blow if the second wave materializes and lockdowns are reinstated. As economies open back up, some countries that were able to control the spread of the virus are now experiencing a resurgence of cases. In France and Spain, for example, the number of new infections is higher now than it was in March during the peak of the first wave.
Failure to control the coronavirus has put economies and political careers in jeopardy. It’s little wonder why President Donald Trump is under enormous pressure to have a vaccine shipped before Election Day in the U.S. As public confidence in the medical establishment wanes, leading U.S. pharmaceutical companies have pledged that they will not release a vaccine until they are certain of its safety and efficacy. The question of who assumes liability becomes critical to the timing of a vaccine.
Why Vaccines Take Time
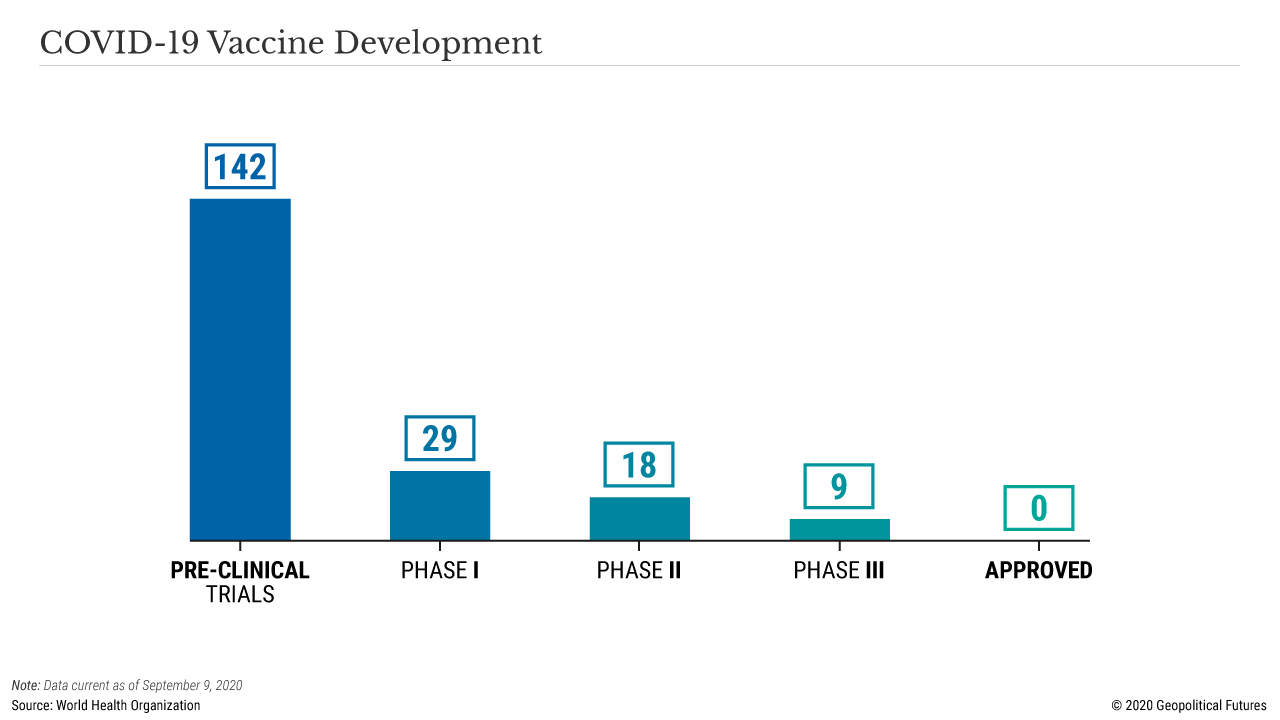
Unfortunately, the process of vaccine testing can only be rushed up to a point. Clinical trials that assess the safety and effectiveness of vaccines take a certain minimum amount of time. No government stimulus or political influence can expedite this process and ensure that basic safety and efficacy standards are met. The reason is that large clinical trials are logistically difficult, often enrolling tens of thousands of volunteers and employing dozens of medical centers across the world.
The testing itself is likewise time intensive. Once volunteers receive their vaccine (or placebo), they are then monitored over the course of weeks or months to determine if they develop the disease or a potentially dangerous side effect. Scientists can reach a conclusion only after enough data has been collected. Occasionally, trials can be cut short if the data is overwhelmingly convincing, but that is not the norm.
Vaccines are not like other pharmaceutical drugs, which are usually administered to patients who are already sick. In the case of a severely ill patient, the primary concern of an experimental drug is efficacy over safety; after all, it doesn’t really matter if the drug harms the patient because he or she is about to die anyway. But the reverse is true for vaccines; because they are given to potentially millions of healthy people, including children, safety is the far bigger concern. Even if the vaccine works, rare but serious side effects could harm thousands of people. (This is one reason that we do not vaccinate against smallpox. The vaccine has killed people. Though it is theoretically a biological warfare agent, the risks of a mass smallpox vaccination campaign greatly outweigh the risk of a bioterrorist attack.)
Other vaccines have also harmed people, or worse. In 1966, a poorly designed vaccine against respiratory syncytial virus killed two kids and worsened the illness in many others. One decade later, a vaccine against pandemic swine flu triggered a rare type of paralysis known as Guillain-Barre syndrome in 450 people. It happened again following vaccination against the 2009 H1N1 pandemic influenza.
Measure Twice, Jab Once
Now liability becomes critical. If a vaccine against the coronavirus produces nasty side effects, it would deal a catastrophic blow to public confidence in major institutions across the board. Everyone has a stake in the production of a successful vaccine. Yet, there are legitimate concerns that a coronavirus vaccine, especially one developed under rushed conditions, may not work as intended. A vaccine developed against the original SARS virus made the disease worse in animal models. And a clinical trial for a coronavirus vaccine produced by AstraZeneca in collaboration with the University of Oxford has been paused because one volunteer is thought to have developed a severe adverse reaction.
It is difficult to overstate the potential damage that a rushed coronavirus vaccine could inflict on confidence in the biomedical community. This is why several major pharmaceutical companies have pledged not to release their respective vaccines until they are shown to be safe and effective by the standards of the U.S. Food and Drug Administration. They may have realized that a premature vaccine deployment is a gamble that may not be worth the financial, reputational and legal risks. After it announced that the clinical trial was paused, AstraZeneca’s stock price fell. Pharmaceutical leaders know that the fallout from releasing a bad vaccine could become an existential threat to their companies and want to avoid being liable for such a scenario.
On the flip side, it is equally difficult to overstate the economic damage of not having a vaccine in the next few months. The longer the pandemic continues, the longer the global economy suffers. The public will become angry, and they will direct their anger toward the institutions they believe failed them. World leaders, therefore, may hope that a vaccine is deployed as soon as possible in order to save their countries’ economies (and, by extension, their own jobs), even if it comes with some risk. Many of them believe the economic consequences do more harm to the population as a whole than the virus. Already being held accountable for economic damage, the governments want the introduction of a vaccine without assuming the liability for that as well.
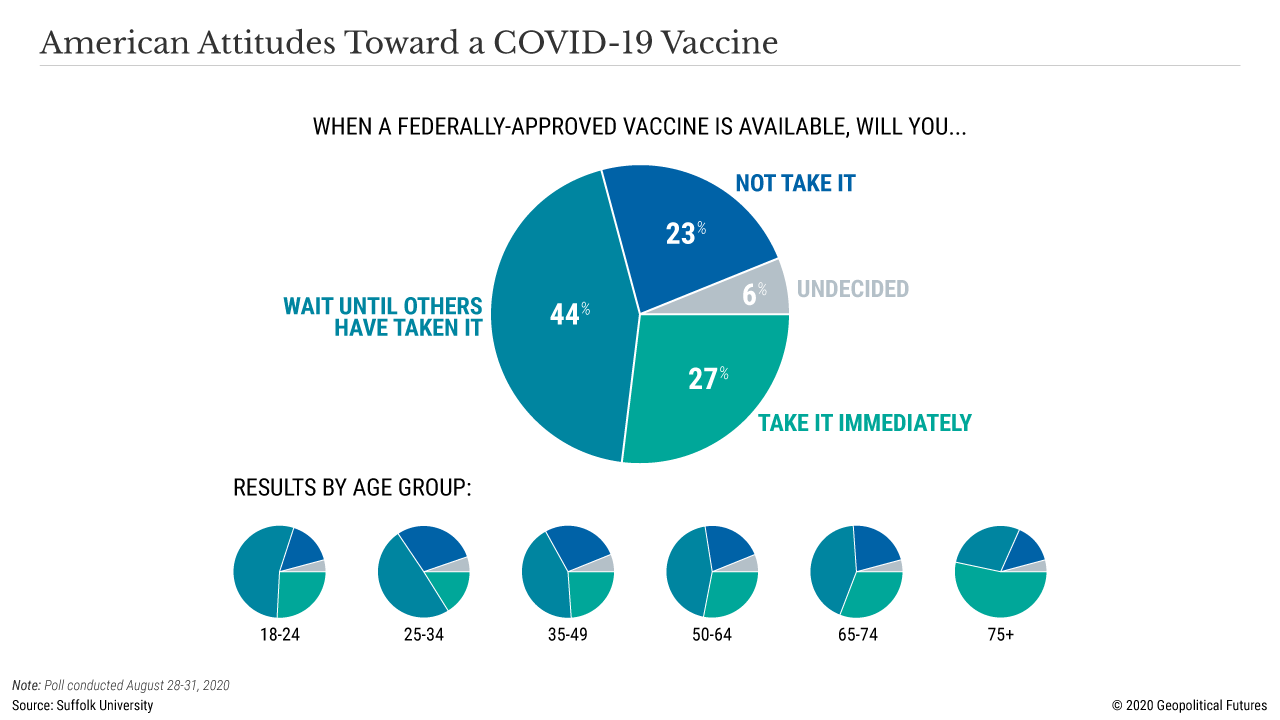
Stuck in between is the general public that simply wants an end to all this. Over the past six months, the public has been bombarded with contradictory information and vociferous debates over shutdowns and school reopenings. Misinformation, particularly on social media, is rampant. While the public trusts scientists and doctors in general, its trust in a coronavirus vaccine is minimal. Two-thirds of Americans say they won’t be first in line to get the vaccine when it comes out. It’s hard to blame them. Just imagine the public backlash if the media depicts children with a vaccine-induced illness because the jab wasn’t tested sufficiently.
One way to interpret the pledge co-signed by several pharmaceutical companies is as an appeal to the government to assume liability. Perhaps behind the message is a subtle hint that they would be willing to release a vaccine that is “good enough” if governments shouldered all the risk. But that’s unlikely to happen. Until the clinical trials are completed, the public will just have to wait while the government and pharmaceutical companies remain in a standoff that runs against an economic clock and mounting social pressure.
No comments:
Post a Comment